Medical Affairs: A Complete Guide for Healthcare Professionals
Many healthcare professionals want to stay connected to the healthcare industry without the grind of clinical practice. Medical affairs makes that possible.
The gap between science and the market is exactly what Medical Affairs is designed to bridge.
Introduction to Medical Affairs
What is Medical Affairs?
Medical Affairs is vital in the pharmaceutical and life sciences industry, which bridges science, patients, and healthcare professionals.
It ensures that therapies are safe, effective, and ethically promoted, while supporting education, evidence generation, and patient-centric care.
Often intersects with regulatory roles such as medical device regulatory affairs to ensure products are safe, effective, and ethically promoted.
Medical Affairs: History and Evolution
The Medical Science Liaison (MSL) position originated in 1967 at the Upjohn Company. Initially, it was done by the sales reps to respond to doctors’ scientific questions.
In the long run, medical professionals were trained to replace it, and the position shifted to Medical Affairs so that no biased information could be given.
By the 2000s, medical affairs expanded to include evidence generation, scientific publications, medical education, and patient engagement.
As treatment becomes more complicated, medical affairs assist doctors, patients, and other interested parties know about real-world safety, effectiveness, and data.
Medical Affairs has become a strategic pillar of healthcare that helps connect science and society with quality medical knowledge.
Now that we’ve seen how Medical Affairs evolved, let’s look at the core functions professionals perform today.
Source: medicalaffairs(1)
Can a BDS Graduate get a Job as a Medical Affairs Professional?
Yes, a BDS graduate can comfortably get a job as a medical affairs professional, particularly in roles such as medical advisor, clinical research physician, or pharmacovigilance.
Read below to know what roles a BDS graduate can transition into.
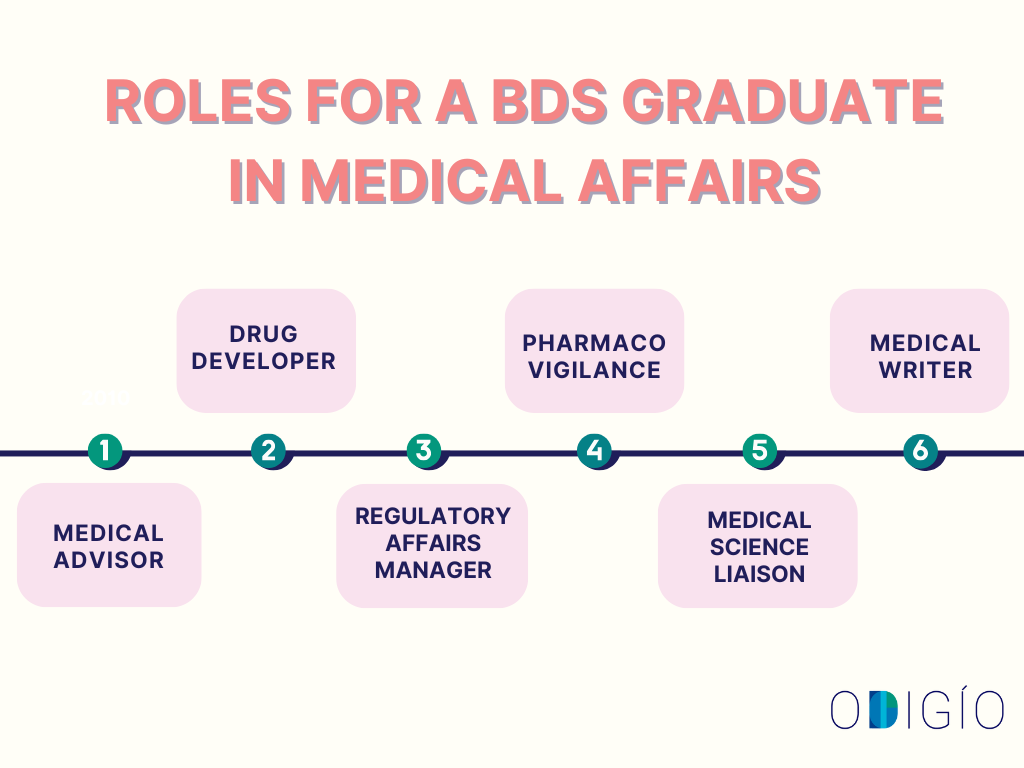
Potential Roles for a BDS Graduate in Medical Affairs
Medical Affairs offers BDS graduates multiple career paths to apply their scientific knowledge beyond dentistry. Read below to know more.
- Medical Advisor: Evaluating clinical data, coordinating medical activities, and advising on healthcare-related aspects.
- Drug Developer: A BDS graduate can help contribute to the development and formulation of new medicines.
- Regulatory Affairs Manager: Focuses on ensuring that the medical products and services are regulatory compliant.
- Pharmacovigilance: Ensures, monitors, and analyses drug safety data to minimise health risks.
- Medical Writer: Dental graduates can work as medical writers, where they write educational content for the medical and pharmaceutical sectors.
- Medical Science Liaison: A BDS graduate who serves as a medical science liaison serve as a bridge between pharma and healthcare professionals by sharing scientific insights, engaging KOLs, and supporting research, clinical trials, and medical education.
Transferable Skills a BDS Graduate Brings to Medical Affairs
As a dentist, you already have a strong foundation that aligns with medical affairs roles.
Your background in clinical knowledge, patient communication, and scientific understanding directly transfers to this field.
Skills like interpreting:
- Research
- Explaining complex concepts in simple terms, and
- Building trust with patients naturally translates into engaging with healthcare professionals and Key Opinion Leaders (KOLs).
In addition, your familiarity with clinical practices and evidence-based medicine equips you to support clinical trials, pharmacovigilance, and scientific exchange with credibility.
Career Path for BDS Graduates: Qualifications & Training
To transition into medical affairs, BDS graduates can strengthen their profile with these additional qualifications, certifications, and industry exposures :
- Postgraduate courses – Clinical Research, Public Health, or Healthcare Management
- Certifications – Pharmacovigilance, Regulatory Affairs, Medical Writing, Drug Safety
- Workshops & training – Scientific communication, Evidence-based medicine, Clinical trials
- Industry exposure – Internships in pharma, research organisations, or CROs
- Networking – Attending medical conferences, joining professional associations
What are the Key Functions of Medical Affairs Professionals?
Medical affairs professionals play a bridge between the company and the medical community.
The key functions of a medical affairs specialist are:
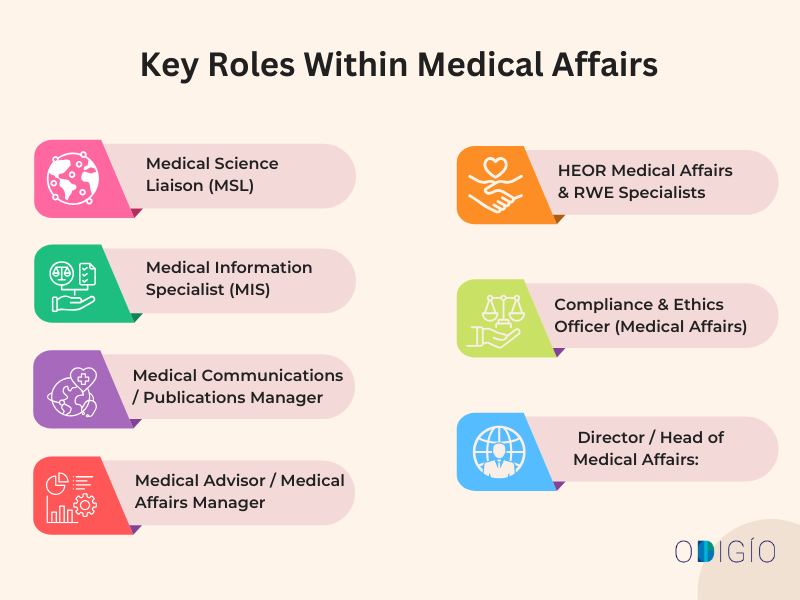
What are the Key Roles Within Medical Affairs?
The key roles within the medical affairs (MA) can be included in the 5 main categories:
- Field Facing Opportunities
- Medical Science Liaison (MSL):
- An MSL is a specialist with advanced scientific training. Their main role is to maintain relationships with Key Opinion Leaders (KOLs)
- Medical affairs MSL provides an external stakeholder link, bridging the gap between clinical development and commercial success.
- They collect credible insights that help product development.
- Medical Science Liaison (MSL):
Source: iqvia(2)
- Scientific Communication & Information Roles
- Medical Information Specialist (MIS):
- Pharmaceutical medical information is a broad term for specialists collecting product data for on and off-label use.
- MIS also collects data and creates fair, medically backed information for patients and healthcare professionals, including publications and product descriptions for the committee.
- Apart from this, the MIS also answers questions related to a specific drug through verbal telephonic conversation.
- Attend medical congresses and scientific meetings to enhance knowledge and provide face-to-face scientific exchange.
- This role is typically for nurses or pharmacists with drug information residency backgrounds.
- Medical Communications / Publications Manager
- Their role is to develop manuscripts, abstracts, posters, and presentations.
- They act as a bridge between the pharmaceutical clients, clinical experts, and medical writers, ensuring all publications are scientifically accurate, compliant with industry standards, and delivered on time.
- Strategic & Cross-Functional Roles
- Medical Advisor / Medical Affairs Manager
Below is the difference between a medical advisor and a medical affairs manager.
| Aspect | Medical Advisor | Medical Affairs Manager |
|---|---|---|
| Primary Focus | Provides scientific expertise and clinical guidance for a product or therapeutic area. | Oversees medical activities and ensures alignment of projects with organisational goals. |
| Scope of Work | Engages with KOLs, supports clinical trials, gathers medical insights, and participates in evidence generation. | Manages cross-functional teams, coordinates medical strategy execution, and supervises operational activities. |
| Decision-Making | Advises on clinical and scientific matters; supports evidence-based decisions. | Makes managerial decisions regarding resources, timelines, and project prioritisation. |
| Field Engagement | Often interacts with healthcare professionals and KOLs for scientific exchange. | Mostly office-based; may interact with teams and stakeholders rather than field HCPs. |
| Strategic Role | Focused on clinical/scientific strategy for therapeutic areas. | Focused on operational and tactical implementation of medical strategy. |
| Reporting | Typically reports to the Medical Manager or the Head of Medical Affairs. | Typically reports to the Head of Medical Affairs or the Medical Director. |
- HEOR Medical Affairs & RWE Specialists
- The main goal of health economics and outcomes research (HEOR), real-world data (RWD), and real-world evidence (RWE) specialists is to analyse health data for better outcomes.
- These specialists are mainly hired in the following sectors:
- Pharmaceutical and biotech
- Clinical research organisations
- Consulting firms
- Government and public health organisations
- Compliance & Governance Roles
- Compliance & Ethics Officer (Medical Affairs): The specialists ensure that the pharma and biotech companies abide by the regulatory standards for all medical affairs activities, such as:
- Scientific communications
- Educational materials, and
- Stakeholder engagements
- Key responsibilities are:
- Developing policies
- Conducting training and audits
- Monitoring regulatory changes
- Compliance & Ethics Officer (Medical Affairs): The specialists ensure that the pharma and biotech companies abide by the regulatory standards for all medical affairs activities, such as:
- Leadership Roles
- Medical Affairs Director / Head of Medical Affairs: They oversee the team, budgets, and alignment with patient and organisational goals.
- Apart from this, they define the overall medical strategy.
Medical Affairs Salary: What Healthcare Professionals Can Expect
The salary of a medical affairs professional depends on the level of experience, role, company size, and geographic location.
The base pay ranges between 6L and 9L per year(3).
The Future Scope of Medical Affairs for HCPs
The future scope of medical affairs for healthcare professionals (HCPs) lies in real-time, authentic access to scientific data and patient-centric solutions.
With advanced technologies and a diversion towards AI, medical affairs will provide HCPs with deeper insights, better education, and support in making informed treatment/medical decisions.
With their scientific and patient-care expertise, clinicians can build rewarding careers in areas such as:
- Leadership Roles: Becoming a medical Director or Head of medical affairs, shaping strategy, and guiding cross-functional teams.
- Research & Evidence Generation: Leading real-world evidence studies, post-marketing research, and clinical trial support.
- Medical Education & Communication: Training healthcare professionals, developing scientific content, and presenting at medical congresses.
- Strategic & Advisory Roles: Collaborating with regulatory, market access, and commercial teams to align medical insights with business goals.
The Role of the Medical Affairs Professional Society (MAPS)
The Medical Affairs Professional Society (MAPS) is the largest global nonprofit organisation dedicated to advancing the medical affairs function in the life sciences industry. Its role is to:
- Professional Development: Provide education, training, and resources for medical affairs professionals across pharma, biotech, and device industries.
- Best Practices & Standards: Develop and share global standards, frameworks, and best practices for the evolving role of medical affairs.
- Networking & Collaboration: Create a community where medical affairs professionals can connect, share knowledge, and learn from peers and industry leaders.
- Thought Leadership: Publish position papers, guidelines, and insights that shape the future of medical affairs.
- Events & Conferences: Organise global and regional conferences, webinars, and workshops to promote learning and innovation.
- Career Growth: Offer certifications, mentorship, and career resources to help members grow professionally.
What is the Difference Between Medical Affairs and Regulatory Affairs?
Go through the table below to understand the difference between medical and regulatory affairs.
| Aspect | Medical Affairs | Regulatory Affairs |
|---|---|---|
| Main Focus | Scientific exchange, evidence generation, patient engagement | Regulatory submissions, approvals, compliance |
| Key Role | Bridge between the company and the medical community | A bridge between the company and regulators |
| Activities | KOL engagement, clinical trial support, publications, and education | Preparing dossiers, labelling, and post-market compliance |
| Output | Scientific knowledge & real-world data | Legal approval & compliance with health authorities |
| Goal | Improve patient outcomes & knowledge | Ensure safe, legal market access for products |
Success Stories of BDS Professionals in Medical Affairs
At Odigio, we make the impossible possible! Several of our BDS clients have successfully transitioned into a medical affairs role. Their journeys prove that with the right preparation, BDS.
Conclusion
Medical affairs has become the strategic pillar of the life sciences industry.
It offers a rewarding career path for healthcare professionals that blends science, strategy, and patient care without the routine pressures of clinical practice.
The opportunities are vast and growing if you are interested in leadership, research, education, or cross-functional strategy.
As therapies become more complex and technology evolves, Medical Affairs will remain central to advancing science, ensuring compliance, and improving patient lives.
